Development of Composite Phase Change Material (PCM) based Thermal Management System for Battery of Electrical Vehicles

Dr Absar M Lakdawala
Professor, Mechanical Engineering
Institute of Technology
Electric vehicles (EVs) and plug-in hybrid electric vehicles (PHEVs) are promising technologies to help reduce the amount of petroleum consumed for transportation. In both EVs and PHEVs, the battery pack is a key component in enabling their fuel savings potential. The battery is also one of the most expensive components in the vehicle. Temperature is one of the most significant factors impacting the performance and life of a battery. In particular, operating a battery at elevated temperatures reduces its life. It is therefore important to design and implement effective battery thermal management systems.
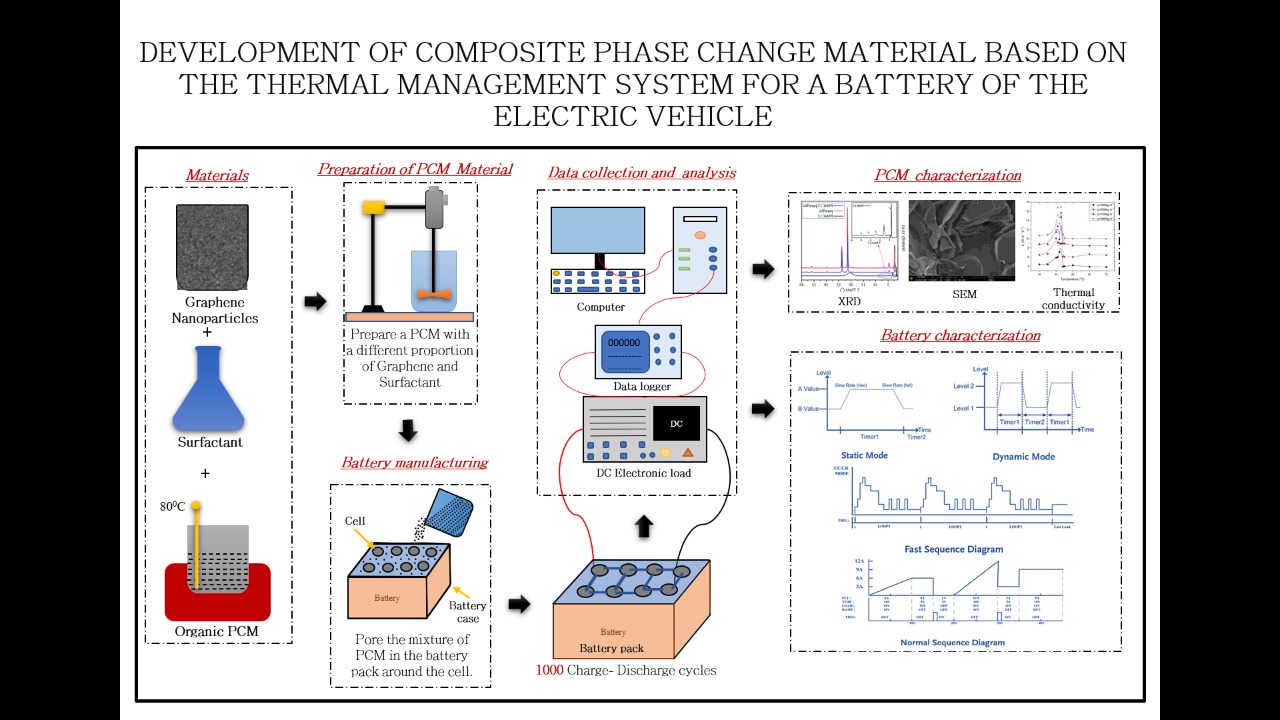
The use of phase change material for thermal energy storage has gained importance in the last decade. In recent years, graphite materials have been used to improve the thermal conductivity of paraffin. In this context, it is essential to identify the “Thermal performance” of the PCM based battery cooling system when subjected to dynamic loading. Further, it is also essential to identify the change in the thermo-physical properties of the PCM during its life cycle.
This proposed work explores whether thermal management using PCM could provide similar benefits in EV or PHEV applications. Vehicle batteries can have duty cycles that are significantly different from those of soldier applications. Therefore, PCM technology should be carefully assessed to determine whether it would improve upon existing vehicle battery thermal management technologies.
While advances in electric car batteries have allowed them to produce more power and require fewer charges, one of the most significant concerns for battery safety remains the ability to build an efficient cooling system. Lithium-Ion batteries in Electric vehicles are more prone to heat during their discharge than a Lead-Acid battery present under the hood in a conventional combustion vehicle. The performance of a Lithium cell is highly influenced by its temperature; Cells experience the Goldilocks effect, in which they do not work well during either extreme cold or extremely hot temperatures, which can result in permanent and serious cell damage or rapid deterioration. The operating temperature has a significant impact on the battery’s lifetime. Temperature control methods that are effective contribute greatly to battery health and lifespan. Furthermore, as the capacity and charge/discharge rate of batteries rise, battery security becomes increasingly important.
Working on the principle of the voltage difference, the electrons present inside during high temperatures are excited thus reducing the overall potential difference between the two sides of a battery. The internal resistance of a cell greatly depends on the temperature of the cell and when there is a significant internal temperature fluctuation, differing charge and discharge rates for every cell might occur, resulting in poor battery pack performance. As a result, in conjunction with cooling, heating of the cells may be necessary at lower ambient conditions to avoid cell damage during the rapid charging when the battery pack is too cold; this is because the internal resistance of the cells increases when they are cold. When the temperature of a lithium battery cell falls below 5°C, it cannot be charged quickly, and it cannot be charged at all when the temperature falls below 0°C. When the temperature of lithium batteries rises over 45°C, they begin to deteriorate quickly.
The chemical processes that take place inside a battery are affected by rising temperatures. Higher temperatures have several consequences for lithium-ion batteries, including improved performance and storage capacity. According to several scientific reports, increasing the temperature from 77 to 113°F resulted in a 20% improvement in maximum charge capacity. As the battery’s performance improves, the battery’s lifespan shortens over some time. With the decreased lifetime it becomes difficult to preserve the range of an electric vehicle for a larger period. Thus, it is ideal to keep the battery pack operated within the optimum temperature range. Cooling systems must be able to maintain a temperature range of roughly 20-40°C within the battery pack, and also the lowest temperature difference inside the battery pack.
The phase change materials (PCMs) applied as passive heat sinks have attracted a lot of attention. Latent heat storage – using phase change material is one of the most efficient ways of storing thermal energy. Unlike the sensible heat storage method, the latent heat storage method provides a much higher storage density, with a smaller temperature difference between storing and releasing heat. Materials to be used for phase change thermal energy storage must have a large latent heat and high thermal conductivity. They should have a melting temperature lying in the practical range of operation, melt congruently with minimum sub-cooling and be chemically stable, low in cost, non-toxic, and non-corrosive. Materials that have been studied during the last 40 years are hydrated salts, paraffin waxes, fatty acids, and eutectics of organic and non-organic compounds. Depending on the applications, the PCMs should first be selected based on their melting temperature. Materials that melt below 150C are used for storing coolness in air conditioning applications, while materials that melt above 900C are used for absorption refrigeration. All other materials that melt between these two temperatures can be applied in solar heating and heat load leveling applications.
Commercial paraffin waxes are cheap with moderate thermal storage densities (~200 kJ/kg or 150 MJ/m3) and a wide range of melting temperatures. They undergo negligible sub-cooling and are chemically inert and stable with no phase segregation. However, they have low thermal conductivity (~0.2 W/m 0C), which limits their applications. Metallic fillers, metal matrix structures, finned tubes, and aluminum shavings are used to improve the thermal conductivity of the paraffin waxes.
Dr Absar M Lakdawala said, “In the present research, graphite nanoparticles are suggested as thermal conductivity enhancers for the paraffin waxes based PCM. The main novelty of the present research is to improve the thermo-physical properties of organic PCM by adding graphite nanoparticles. The resulting composite PCM will be analyzed for thermal performance as well as cyclic heating and cooling to check its feasibility to work as a thermal battery management system for an electrical vehicle. A charged thermal battery will have at its disposal ~170 kJ/kg of energy which can be used for various applications.”
Note: Dr Absar M Lakdawala is the Principal Investigator (PI), and Dr Dhaval Shah and Dr P N Kapil (Assistant Professors, Institute of Technology, Nirma University) are the Co PI for this research project.